The Disappearing Spoon Chapters 18 and 19
Chapter 18 was rather a crazy mix of ideas and stories, not saying that the majority of the book hasn’t been exactly that. Anyways, the chapter starts off with talking about the NIST and the BIPM, two bureaus that focus on being as precise with all measurements as possible. The author talks about the most cherished of all measurement tools, the kilogram. The International Prototype kilogram was a cylinder that weighed EXACTLY one kilograms and scientists went to absolutely ridiculous measures to keep it this way. However, when it started to shrink mysteriously, they gave up this method to use an easier and more precise one… email. Then the details start with all of the headache-causing examples and explanation but from what I understood is that scientists use a cesium run clock in order to calculate the absolute most precise measurements of time that they possible can. Ten the chapter moves into the section dealing with constants. Basically, the world is how it is because of alpha being exactly how it is, without ay variation. Because alpha is something measurable, scientists of course found the exact measurement. Then came the data from Oklo, a site in Africa where the only known fission reactor exists. Based on research done, some scientists guess that alpha is slowly getting bigger and in return, there was a variation in data. This inconstant, if true, means that the big bang theory would be impossible. Then Australian ideas are introduced with quasars (or black holes) and this somehow led to the theory of a fundamental constant changing. The final ending to this chapter deals with Fermi and his question of whether other life exists or not. Based on the Drake Equation, much life could exist. However, we will never find out unless we take the initiative. Aliens are being lazy I guess.
Finally the last chapter! And it was actually fairly good too. It started with the introduction of the most rare element known on Earth, astatine. The next rarest element in francium, which can be explained through a long set of decayed elements and probabilities. After scientists were playing with heavy elements and stability, they came up with the idea of the “island of stability”. However, this theory basically rests on francium and now to stabilize the nuclei of elements. Based on this theory, scientists have been able to fill in the last rows of the periodic table with hypothesized elements all dealing with latin names. However, the real question to many scientists was what the limits of the periodic table are. The chapter then moves into the periodic table and how it is set up dealing with properties. Over the years there have been hundreds of ways of organizing the elements and the author shows a genuine interest and respect for the ingenuity of a lot of them, especially some of the 3D ideas. I didn’t quite understand the part about jellium and stay electrons and such, but overall it was a very interesting end to a great chapter and an even more intriguing book. I’m happy with it. (:
Unit 4 Review – Toxins
Unit 4 focused on the topic of toxins and their role in chemistry. Lesson 1 was the introduction into toxins and their effects. Toxins are substances that interact with living organisms and cause harm. The beginning of the unit was mainly teaching us about chemical equations and how to interperet, write and balance them. Within these chemical equations we learned about the difference between physical and chemical changes. A physical change is a change in matter in which a substance changes form but not identity while a chemical reaction is a change in matter where it results in a new substance or new properties (changing the chemical composition). Dissolving is considered a physical change. One important thing that we went over is that matter cannot be created nor destroyed, so the amount of matter should be equal on both sides of a chemical equation. However, if a product in a chemical reaction is a gas and is not contained, then the mass will be less on the right of the equation. This led us to balancing equations using the coefficients of elements, or the number that refers to the number of atoms you have because you cannot change the subscripts of the compound/element. The types of reactions that we were working with can be classified into the four main types of reactions: combination, decomposition, single exchange and double exchange reactions. Then we actually got into toxins and lethal dosages. The lethal dose of a substance is considered the LD50 of a substance. This refers to the amount of an ingested substance that kills 50% of a test sample of animals. This amount depends on the amount of toxic substance and the mass of the organism. One important lesson we skimmed over was on percent error (equation below). We then learned that the mass of one mole of atoms of an element in grams is numerically the same as the average atomic mass for that element. This is also where we practiced scientific notation, or writing numbers in a simpler form to work with. From here is where we started working with conversions, such as finding the amount of moles in a certain mass of an element or the amount of grams of a certain element in a chemical compound using moles, molar masses, and the wonders of a conversion table.
In the next section we learned more about what was actually in the solutions that we were playing with. First of all, we have a solution, or a mixture of two substances that is uniform throughout. Then we separate this into a solute and solvent, or the two substances that make up the solution. The easiest way to remember that a solute is what is put into the solvent is the phrase “you can put a flute in a vent”. Then we focused on concentration and morality, which are basically the same thing. However, concentration is a measure of the amount of solute dissolved in a certain volume of solution. Molarity is the the concentration of a solution expressed in moles of solute per liter of solution. We learned more about concentration from the last lesson in that it does not depend on the size of the sample. There should be an even distribution of particles in a solution. Therefore, if you have one type of solution, perhaps sugar water, and you poor the solution into two different containers then both solutions will share the same concentrations. We then learned how to calculate the number of moles of particles by multiplying the volume and molarity of the solution. However, it’s good to remember all of the ions in the formula when calculating the amount of moles in an ionic solution, such as salt in water. The next section started up with pH.
An indicator is a molecular substance that changes color when it comes into contact with an acid or base. The indicator’s color change is then shown on the pH scale. The substances on the left of the scale, or from 0 to 7, are all acids. The substances on the right side of the number line are called bases, which are from 7 to 14. The substances in the middle, or around 7, are called neutral substances, which means they are neither acids or bases. Acids and bases are therefore classifies by their observable behavior, such as color change because they change the color of an indicator. After learning about acids and bases we started in on the logarithmic relation of pH. The major point is that pH is related to [H+] by the formula: pH = -log[H+]. Therefore, the pH scale is a logarithmic scale that describes the concentration of H+ ions in solution. We then learned that the pH of water is neutral, and therefore the pH scale number is 7. The molarity, [H+] and pH are all related for every solution. One important detail to remember is that the greater the concentration of H+ ions, the lower the pH of the solution and the more acidic it is. Another important relation to realize is that [H+][OH-] = 1.0 x 10-14. The next lesson was about titration. A titration is a procedure in which a neutralization reaction is monitored with an indicator. When the indicator shows that the equivalent point (or point at which moles of base added have neutralized acid) in a titration between a strong acid and strong base, the number of moles of H+ ions equals the number of moles of OH- ions. We then finised the unit by learning about conversion using the mole tunnel and also solid precipitates. (: Such a long section! But good luck for the final, and have a wonderful break! (:
– Balance the following chemical equation: Zn + HCl —> ZnCl2 + H2
* Zn + 2HCl —> ZnCl2 + H2
– How many grams of solute are dissolved in a 500.0 mL sample of 0.50 M sodium sulfate?
* 0.50 M = x / 0.5 L x = 0.25 mol Na2SO4. 0.25 mol x 142 g = 36 grams
– How would you make a 1.0 M solution of silver nitrate.
* I would first find how many moles of silver nitrate I would need by multiplying 1 liter by a 1.0 molarity and then calculate how many grams of silver nitrate is in one mole and add it to 1 liter of water.
Disappearing Spoon Chapters 15, 16, and 17
I actually really liked chapter 15. I’ve always had an interest in “mad scientists” and their supposed ways but this chapter really dug deep into actual scientists who shared “mad” characteristics. The chapter first starts off with the story of William Crookes. The story explains how he was a rather well acknowledged scientist in his early thirties. However, when his brother died at sea he apparently went “mad”, or at least that’s what a few people thought. He made contraptions and theories of spirituality and the existence of his dead brother when he went to séances en mass to mourn for his brother. Although many scientists thought of his work as ridiculous ghost ideas, he managed to pull out of his pathological science stage and began woking with selenium and radioactivity. Nicely following after the author provides a much appreciated definition of pathological science. He then goes on to tell the story of scientists finding shark teeth on the bottom of the ocean covered in manganese. Then the theory of the megalodon starts up. Just as a side note, I am deathly scared of sharks and this part of the chapter didn’t help with that fear at all. Thankfully, the story moves from giant scary sharks to the scientists Pons and Fleischmann. These two scientists unfortunately succumbed to fame and credit over their scientific findings with electric currents, water and palladium. When they discovered that palladium soaks up an unimaginable amount of hydrogen when in water with electricity, they automatically released their results of a new age of energy before they could test the experiment more thoroughly. Thy went down in history as frauds of their own results but also as the scientists who started the important research on such a reaction. The next story of Röntgen is a bit more happy and successful. Without the crazy details, he basically skirted pathological science madness and was still able to manage creating the x-ray. His research claims him to be a hero in history. (:
My basic summary of chapter 16 is just a headache in text. It was extremely interesting but so hard to comprehend due to the “uncertainty” of it. The majority of the chapter dealt with the crazy characteristics of elements when exposed to extreme conditions of temperature. Kean starts out with the story of a few Englishmen that set out to be the first men to ever be 90 degrees below the equator. Sadly, norwegians had already been there first when the five men reached the spot. Matters got even worse for the men because they became stuck in snow storms for weeks and ran out of supplies, including a heat source. This is due to the fact that their tin kerosene holders leaked. This is thought to be the effect of a possible “tin “leprosy”, in which the atomic structuring of atoms in the tin becomes weak and crumbly at low temperatures. The next part of the chapter doesn’t focus on certain scientists like the previous chapters but more so the fascinating forma that matter can take when temperature is either extremely cold or hot. The first example is of when scientists dropped the temperature to around – 445 degrees Fahrenheit in order to combine xenon and argon as well as krypton to other elements, including the noble gases. The next example was a bit hard for me to follow but it talked about lasers and masers, a rather intriguing and all too real thing. Using a crazy method of electron moving/jumping and rearranging, scientist Townes was able to create a maser. However, as basic as that was, the author then moves on to an even more unimaginable idea of Bose-Einstein matter. This then moved into an experiment conducted that reached one billion of a degree above zero and created an all too new toe of matter where atoms basically combine into one huge atom. One far out chapter but interesting enough.
I can’t say that chapter 16 was any less confusing but it did focus on one of my favorite things: bubbles. However, this chapter really made me have a strong appreciation of bubbles other than just fun things to play with. The chapter starts out with the interesting story of Donald Glaser, who came to the idea of using bubbles for some current experiments going on by scientists studying exotic fragments of neutrons, electrons and protons. Watching his beer glass and bubbles, Glaser was able to take part in the creation of the “bubble chamber”. The chapter then moves onto smaller stories of the Roman’s love of calcium bubbles and the use they could provide. Rutherford is then introduced as basically that of a troll but based on his radioactive experiments he became famous. He and another scientist, Soddy, were able to prove that elements could mutate into other elements. He then used this knowledge and discovered helium particles being produced from this decay as bubbles. This theory then went on to be used as a dating device to help find the age of the earth by measuring the amount if helium trapped in rocks (uranium decay). The next story is then of a scientist by the name of Putterman. He had the idea of sonoluminescene work and soon began a small experiment that led to his discovery and most of the discoveries in the latter part of the chapter were based on the research of foam. Who knew bubbles could be so epic in elemental history.
Lessons 25 and 26
Lesson 25 – Mole Tunnel
In this lesson we learned to use gram-mole conversions to solve stoichiometric problems. The main idea of the “mole tunnel”, or way of solving stoichiometric problems, is to: 1) convert grams to moles 2) fine the molar ratios 3) convert moles to grams. Usually, you will need know the grams of reactant you need to produce a certain number of grams of product when working with chemical equations. Therefore, when using the mole tunnel, you determine the mass of the product produced by a certain mass of reactant by converting mass to moles and then back to mass. Here are some practice problems to help explain a little better. (:
– Explain why you need to do gram-mole conversions when carrying out chemical reactions.
* You need to have moles as your unit of measurement because you need to use the molar ratio when solving problems.
– Consider this balanced chemical equation: 2Na2CO3(aq) + CaSO4(aq) —> Na2SO4(aq) + CaCO3(s)
1) Suppose you want to make 30.0 g PbCl2. How many grams of NaCl do you need? 12.6 grams NaCl
Lesson 26 – Get the Lead Out
This lesson was about how to find the limiting reactants of a chemical reaction as well as how to solve limiting reactant problems. We also learned how to calculate percent yield. As a general definition, a limiting reactant is what determines how much product you can make from a chemical reaction. Using this new term, we learned that you can follow some easy steps to solve a limiting reactant problem. First, balance the equation and find the molar mass of each compound. Next you need to use the molar masses yo determine the number of moles of reactant you have and then you can use the mole ratio to find the limiting reactant. Using the limiting reactant you just found, you can now calculate the maximum amount of product. We then learned about percent yield. Here are some practice problems to help explain. (:

– What is percent yield?
* Percent yield is the comparison of the theoretical yield and the actual yield, or of the product produced with a limiting reactant compared to that of what is produced when the reactionis actually carried out.
– Silver nitrate, AgNO3 reacts with sodium chloride, NaCl, in aqueous solution to form solid silver chloride, AgCl(s), and aqueous sodium nitrate, NaNO3(aq). Suppose you start with 6.3 g of AgNO3 and 4.5 g of NaCl.
1) Write a balanced chemical equation for this reaction.
* AgNO3(aq) + NaCl(aq) —>; AgCl(s) + NaNO3(aq)
Lessons 23 and 24
Lesson 23 – Solid Evidence
In this lesson we learned about precipitation, specifically of solids, and solubility. A precipitate is the solid created when a reaction in an aqueous solution forms a compound that is not very soluble. However, when analyzing precipitation reactions, some ionic solids are more soluble than others. When a compound reaches the limits of its solubility, an undissolved solid is now visible. When we talk about solubility, it means the degree to which a compound dissolves in water. A few things to keep in mind is that precipitation is not limited to solids, such as the process of rain being one related to liquids and gases. Also, precipitation reactions are usually double exchange reactions. Here are some practice problems to help explain. (:
– Write a balanced equation for the following equation: NaCl(aq) + Hg(NO3)2(aq)
* 2NaCl + Hg(NO3)2(aq) —> 2NaNO3 + HgCl2
– Explain what a spectator ion is.
* A spectator ion is an ion that appears on both sides of a complete equation but does not directly participate in the reaction.
Lesson 24 – Mole to Mole
This lesson focused on finding the moles of product in a reaction as well as what limiting and excess reactants are. In order to learn about these, we had to learn about mole ratios. A mole ratio is the ratio presented by the coefficients in a chemical equation showing how many units of each substance must combine to make the maximum amount of product. You can just look at the coefficient to determine the molar ratio. The main idea of excess and limiting reactants in that when reactants are not combines in their exact mole ratios, one of the reactants will run out (limiting) and one will be left over (excess). Therefore, in order to get the maximum amount of product from a reaction, reactants must be mixed in the correct proportions. So when you compare two things in a balanced equation, always use moles! Here are some practice problems to help. (:
– Aqueous silver nitrate reacts with aqueous sodium chloride producing a precipitate of silver chloride. AgNO3(aq) + NaCl(aq) —> AgCl(s) + NaNO3(aq)
1) How many moles of NaCl do you need to react with 0.10 mol of AgNO3? 0.10 mol NaCl
2) How many grams of NaCl does this represent? 5.8 grams NaCl
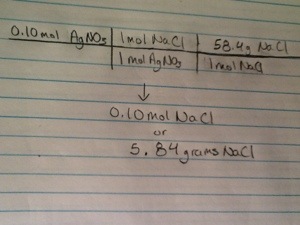
– Explain how to create the maximum amount of product from a reaction.
* In order to create the max amount of product, you need to mix the reactants in the correct proportions, just like following a recipe. (:
The Disappearing Spoon Chapters 13 and 14
Chapter 13 is the chapter I inevitably knew had to be in this book. Humanity circles around money, wealth and value. Sad, yet true. This chapter starts off talking about an a prince by the name of Midas. It goes on to share his reign and the gold and bronze age, seeing as to how the people of 3000 BC could not recognize the difference between bronze and other metals with zinc in them. This introduced the idea of counterfeiting, which was the main relation between the elements in this chapter. The story then moves onto gold (skipping silver for later and l know not why) and starts with gold rushes. Here there was an actually interesting story of a huge gold rush in Australia in which gold was so easily accessible that you could just pick it up off the ground. However, once the population boomed and mining became the priority along with building to supply the growing need for housing, it became apparent that resources had huge inflation prices. To top it off, the rocks they were “throwing away” from mining and building with happened to contain the most gold themselves, making deconstruction a wide skill (sarcasm). Then the chapter moves onto what I recognize most and that is paper money. Starting in China, Kean moves into talking about counterfeiting techniques and how unique and a pain it would have to be in order to counterfeit an EU bill. We then go into the story of such a valuable metal as aluminum. Aluminum was once a precious metal, valued over gold itself. However, once a scientist was able to find a way to extract pure aluminum, the market for the metal crashed and it is now used as soda cans. Blatant ending to the most well applicable chapter.
Chapter 14 was a bit unexpected for me, but still interesting enough. This chapter focused on the aristocracy, or wealth related skills, that took place in science. To further explain, Kean described this idea simply as only the rich could afford to study science. He then starts talking about Goethe, an author he learned about by one of his college professors. Goethe wrote stories about elements and science that really had an impact on Kean due to his prior opinion of the author. The chapter then moves onto him choosing his partner, Döbereiner, to help him with his studies. The partners were both very successful and even helped to organize the periodic table even further. Döbereiner even came up with the first lighter product, making him possibly even more famous than Goethe. The chapter then starts on the story of Moholy-Nagy, a man which had the most correct theory of business in that people always want the newest product, no matter how well the old product worked. Then came the pen. I found it hilarious how obsessed the current population was on the simple pen, mastered by Kenneth Parker. The Parker 51 pen, with all it’s fancy looks seems a bit over the top to me but I suppose I should never doubt the will of Americans. Then came the part that surprised me the most. I never really knew that Mark Twain wrote any literature dealing with science (maybe my own large ignorance). He helped publicize the type writer (which he hated) and not so much the pen (which he loved). Lowell was the next artist and the last discussed, unfortunately. Apparently he was a bipolar poet and artist with a horrible social life. However, when he was tested with lithium to help with his “problem”, he was able to be one “cured”. However, his artwork was arguably never the same. Sad day.
Lessons 21 and 22
Lesson 21 – Neutral Territory
In this lesson we focused on neutralization reactions and how to predict the products of a neutralization reaction. Basically, a neutralization reaction is between a strong acid and a strong base in an aqueous solution. This type of reaction produces an ionic compound (salt) and water and is considered a double exchange reaction. This is because it exchanges cations during the reaction. Another important idea to remember is that when strong acids and bases mix, the pH of the product approaches 7 at 25 degrees. One exception to this neutralization is that when an acidic solution (very concentrated) mixes with a basic solution (not very concentrated) there will be leftover H+ ions after they mix because there will not be enough OH- ins to neutralize all of the H+ ions. That is mainly all of the big details for this lesson, so here are some practice problems to help explain further. (:
– Predict the products of the following equation and balance it: HCl + Mg(OH)2
* HCl + Mg(OH)2 —> MgCl2 + H2O
– Describe two ways to make a strong acidic solution safer.
* One way is to add water to increase the pH to nearly 7, or around neutral. You could also achieve this safer pH by adding a basic solution to the acidic solution to increase the pH to neutral or near it.
Lesson 22 – Drip Drop
We learned all about titrations in this lesson. We learned about the particle views of titrations (in the book) as well as the calculations that accompany the procedures. A titration is a procedure in which a neutralization reaction is monitored with an indicator. When the indicator shows that the equivalent point (or point at which moles of base added have neutralized acid) in a titration between a strong acid and strong base, the number of moles of H+ ions equals the number of moles of OH- ions.
– What is the role of an idicator in titration?
* An indicator is used to help monitor the neutralization reaction .
– How many mL of 0.1 M NaOH would be required to neutralize 2.0 L of 0.050 M HCl?
* 0.050 M = x / 2.0 x = 0.1 mol 0.1 M = 0.1 mol / x x = 1 L = 1,000 mL
Lesson 20 – Watered Down
In this lesson we learned about diluting acids and what happens as you approach a pH of 7. We learned that by adding water to a solution, or diluting it, you can make an acid or a base less acidic or basic. However, an acid can never be made into a base by diluting with water, nor can a base ever be made into an acid by diluting with water. Through our experiment of continually diluting hydrochloric acid, we learned that by diluting an acid, the H+ concentration will decreases and the pH becomes closer to 7. When a base is diluted, the OH- concentration decreases, meaning the H+ concentration increases and the pH decreases to be closer to 7. One last detail is that each time the H+ concentration is diluted ten fold, the pH goes up one unit. Here are some practice problems to help explain. (:
– Explain why you cannot turn an acid into a base by diluting it with water.
* Water has a pH of 7 so the most basic you could get an acid is only close to 7 and no higher.
– Imagine you have 0.75 L of a 0.10 M HCl solution.
1) How many moles of H+ are in the 0.75 L?
* 0.10 M = x / 0.75 L x = 0.075 mol
2) If you add 0.35 L of water, what is the new concentration of the solution?
* 0.075 mol / (0.35 L + 0.75 L) = 0.068 M
3) What is the pH after adding the 0.35 L?
* -log(0.068 M) = 1.2 pH
The Disappearing Spoon Chapters 10, 11, and 12
Chapter 10 was a nice break from the last chapter, full of depression and poison. This chapter focused on medicinal uses of elements. The first element hit on was silver. Using stories such as Tycho Brahe, who lost part of his nose and used a sliver prosthetic one , and other stories such as the pioneers using silver to keep milk from spoiling, the author was able to describe the anti-bacterial properties of silver. He also explains the huge use of copper and silver in plumbing and everyday use for health regulations. Gadolinium was the introduced with it’s huge medicinal contributions die to it’s highly magnetic character. It is used in MRI machines and I found it’s possible use as a cancer cure rather hopeful and it actually led me to doing a little more research, just as I did later when I found out an overdose on silver can make you into a smurf. Then, Louis Pasteur entered the picture. He was the scientist to discover that everything is basically left handed, or at least has a “handedness” to it. He was then able to go on and learn more about chirality. Gerhard Domagk was the next to be talked up. He discovered the great healing powers of prontosil and sparked the huge use of it as a healing agent. Using Pasteur’s discoveries and William Knowles discovery with rhodium, some Parkinson’s patients were able to be “awakened” from their strange mental paralysis. Quite the handful of medical research for one chapter.
Chapter 11 kicked off with a rather gloomy and horrible story of a few astronauts being poisoned with nitrogen gas in a space shuttle while being grounded. Kean describes how it was even a peaceful death because the gas tricks the body into thinking nothing is wrong because the body remains relaxed as long as it breathes out carbon dioxide, a rather eerie concept to me. The author then moves on to a little brighter of a subject and talks about the discovery of titanium prosthetic limbs and the fact that titanium is seen to the body as bone itself and is therefore accepter rather than rejected in surgery and recovery. Then the subject of taste arrises. This part of the chapter really caught my interest because it truly is kind of scary as to how some elements, such as beryllium, can trick someone’s sense of taste so easily. We really do rely on taste like no other and it’s scary to know that something can penetrate that skill so easily. So after thoughts like that, Kean jumps in with a lighter but more political subject of iodine and it’s importance in trying to help decrease birth defects. He describes how several governments iodized salt to help distribute iodine to many people as an easy dosage. However, India did now want to accept the westernization until recently, a rather interesting ending to an interesting story.
The last chapter introduced the new subject of chemistry and politics, but personally I thought it was a horrible love story with some elements and war thrown in there. However, it started off with the story of Marie Skylodowska (Marie Curie), and Pierre. Marie discovered more about the periodic table that eased studying new elements and the hardships that came with them. Later, she then discovered two new elements in the waste of her uranium experiments. Bit the the politics moves in when she named one of the elements polonia, after Poland, in hopes of raising awareness for the poor territory. Kean then talks about her daughter and her own discoveries in radioactivity. Hevesy is then introduced through his use of radium-D and then goes on to explain his career in quantum mechanics and hafnium. Then arises a love story between Lise Meitner and Otto Hahn. They renamed brevium into proactinium because they found that the atoms actually last for thousands of years. Then the story moves into fighting over nobel prizes (which Meitner never ended up receiving despite her HUGE contributions). I found this ridiculously stupid because Hahn, her lover, did absolutely nothing to help mention her. Anyways, I guess every love story has a crappy ending when you’re talking about Nazi’s, chemistry and nobel prizes.
Disappearing Spoon Chapters 8 and 9
Chapter 8 shared the same race to fill the missing periodic table gaps as the last few chapters. However, the beginning of this chapter talked about element 43. Before jumping into the fight for the rights to element 43, the chapter starts with a Time magazine cover with 15 famous scientists. Two of these, perhaps the most important to us in this chapter, happened to be Emilio Segrè and Linus Pauling. Kean shares that the link between these two scientists’s fames are huge mistakes. Element 43 is then discussed and it’s history of having thought to have been discovered by German chemists and other scientists that have all said to have found it. This, as described by Mr. Kean, makes it the Loch Ness Monster of the elements. However, it was not officially “found” until 1937 by two Italians, one being Segrè. Segrè’s mistake was to believe that there were no transuranic elemental properties about element ninety-three and also that he misidentified transuranic neptunium as a fission product. The story then moves onto Pauling and his genius mind, being compared the Leonardo of chemistry. However, even being considered on the the greatest scientists, he refused to think of himself as incorrect when it came to his theory of a tripe helix DNA. He was one of the first scientists to work with and study DNA, but when told his theory did not work my a small colleague, he made the mistake of publishing his idea of the tripe helix. However, some graduate students instead found that DNA is actually a double helix. However, both Pauling and the students received credit. Most of the scientists in the chapter fought for credit rather than the actual discoveries, or at least that’s what I thought.
Chapter 9 was actually rather depressing for me. It starts off talking about “poisoner’s corridor”. This includes elements thallium, lead and polonium. However, Kean begins with cadmium. Cadmium did the most damage in Japan after a mining company kept dumping large amount of the element into the water supply. People began suffering from the disease itai-itai, where they suffered tremendous pain, liver failure and extremely weakened bones. This was caused, after much debate between the mine and a scientist named Hagino, that the rice being farmed soaked up cadmium and therefore poisoned anyone who consumed it. The next poison talked about was thallium and it’s gruesome record in history of poisoning people and killing many. Then there was a nice break from the horror stories of elemental massacres…. punny. Kean talked about the misplacement of bismuth with the poisons because it is actually used in everyday medicines, such as pepto bismol or even the medication prescribed for the symptoms of cadmium poisoning. But the the story moves downhill once again in a rather depressing story of a young boy scout. David Hahn was a young teenager when he began his love of chemistry. However, after his extreme work on a nuclear reactor in his mother’s backyard shed, he was eventually arrested after trying to steal smoke alarms (containing americium, a radioactive element). This was after his young and reckless years of playing with radioactive elements and coming actually coming admirably close to the idea of a working nuclear reactor. A nice sad ending to a terrible chapter.