Lesson 15 – Holey Moley
In this lesson we learned how to create a solution with a certain molarity as well as how to relate mass, moles and volume. We learned that you need some information in order to solve a characteristic of a solution. For example, you can calculate the amount of solid in grams needed to create a solution of a particular molarity by using the molar mass of the solid. Also, you can find the mass of a dissolved solid in a solution if you know the concentration (mol/L) as well as the volume. Here are some practice problems to help explain. (:
– How many grams of solute do you need to make 1 L of the following solution: 0.50 M NaCl?
* 29.3 grams
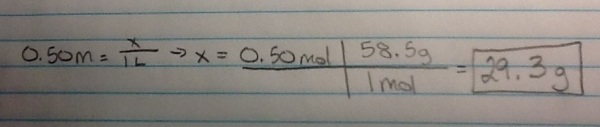
– Explain how you would prepare a solution of sucrose with a molarity of 0.25 M.
* Because the molarity is 0.25, I would first calculate the mass of 0.25 mol of sugar and then add enough water to make the solution 1 liter.
- Posted in: Uncategorized
Awesome as always 🙂
Great job Jenae! I like your organization!